Der Zweck dieses Artikels ist es, die GxP-App-Vorlage als Ausgangspunkt vorzustellen, um Ihren App-Erstellungsprozess zu beschleunigen. Er führt Sie durch die Datenstruktur der App, die wiederverwendbaren Komponenten, die Sie konfigurieren können, ohne Code zu benötigen, und zusätzliche Ressourcen, die Ihnen helfen, ein Experte für die Erstellung von GxP-Apps zu werden.
Datenstruktur innerhalb der GxP-App-Vorlage: Vervollständigung und Tabellen
In dieser Anwendung werden sowohl Tabellen als auch Vervollständigungsdatensätze verwendet, um verschiedene Arten von Informationen zu speichern. Wenn die Informationen für die Wiederverwendung in anderen Anwendungen bestimmt sind, werden sie in einer Tabelle gespeichert. Informationen, die wir nur für Überprüfungszwecke benötigen, werden in den Abschlussdaten gespeichert. Alle Tabellen, die von der GxP-App-Vorlage und den Anwendungen im Composable MES for Pharma verwendet werden, sind unter Verwendung des Common Data Model for Pharma von Tulip aufgebaut.
Vom Composable MES für Pharma verwendete Tabellen
Die Anwendungen im Composable MES for Pharma sind miteinander verbunden und arbeiten mit dem Common Data Model for Pharma zusammen. Das Common Data Model for Pharma bietet einen Ausgangspunkt für die Organisation und Erfassung von Daten in sinnvollen Tabellen, die sich leicht mit neuen Anwendungen erweitern lassen und Ihrem Team helfen, schneller zu skalieren und Herausforderungen zu lösen.
:::(Info) If you'd like to learn more about Tulip's Common Data Model (CDM)
for Pharma, check out this link.
:::
Wiederverwendbare Bausteine in den Anwendungen
Pausieren und Fortsetzen innerhalb von Anwendungen
Die meisten Anwendungen im Composable MES for Pharma können durch eine integrierte Logik wieder aufgenommen werden. Das bedeutet, dass der Fortschritt angehalten und bei Bedarf zu einem späteren Zeitpunkt wieder aufgenommen werden kann.
Jede Prozessanwendung enthält einen Trigger in ihrem Basislayout. Dieser Trigger speichert den Schrittnamen in der Prozessablauftabelle.
Am Anfang jeder Prozessanwendung gibt es mehrere Trigger. Ihre Funktion besteht darin, zu prüfen, ob für die gewählte Charge ein Status "in Bearbeitung" vorliegt. Wenn ein Status "in Bearbeitung" vorliegt, wird die Anwendung an dem Schritt fortgesetzt, an dem sie unterbrochen wurde.
Auslöser für die Navigation
Innerhalb der App-Suite verwenden wir vier verschiedene Arten von Navigationsauslösern: Next, Previous, Go to und Routing Der Trigger Next navigiert, wie der Name schon sagt, die Anwendung zum nächsten Schritt.
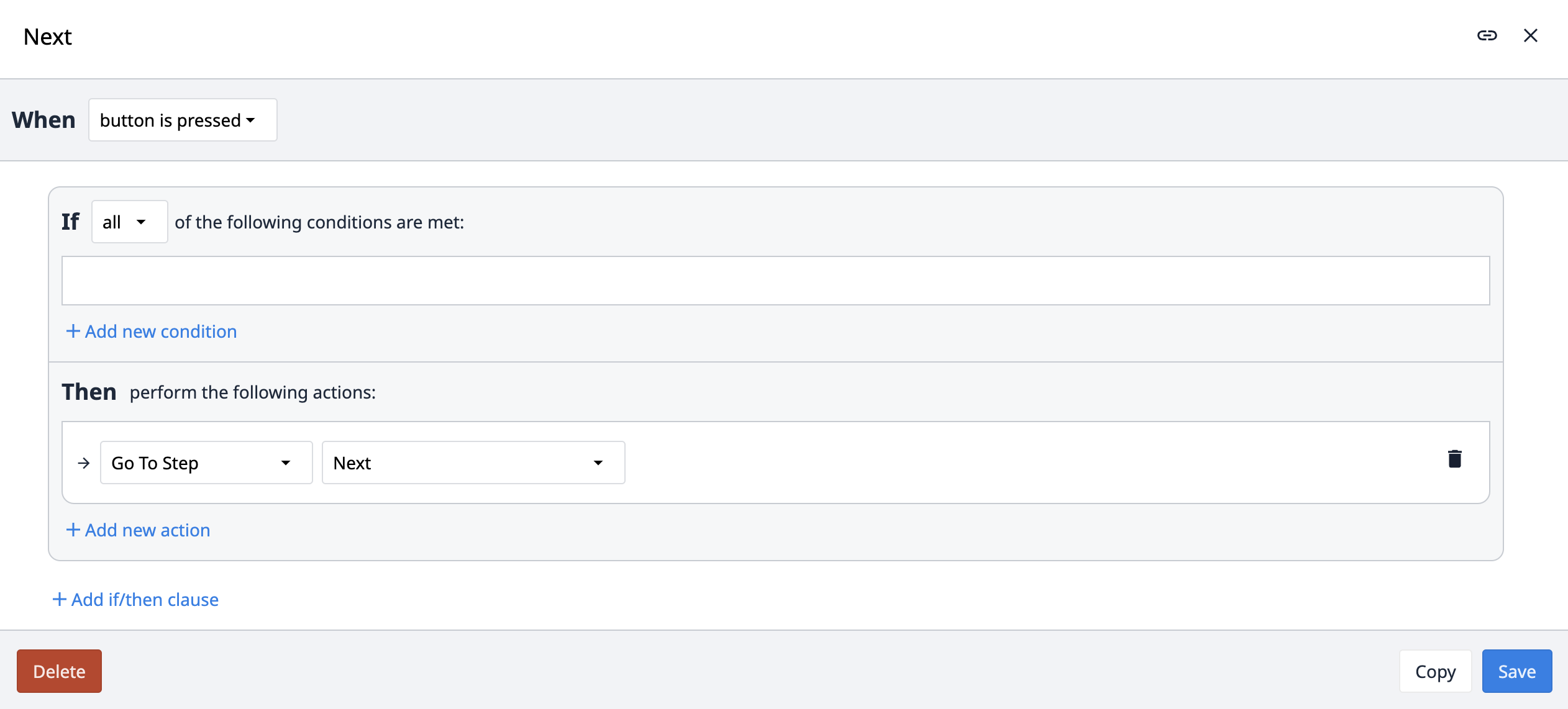
Der Auslöser Previous navigiert die App zum vorherigen Schritt. Statt der Option Previous innerhalb des Triggers verwenden wir jedoch den Namen des vorherigen Schritts. Damit soll sichergestellt werden, dass sich die Navigation zu Previous immer auf die Hauptschrittfolge bezieht und nicht zu einem sekundären Schritt wie z. B. Comment navigiert. Wenn ein Benutzer beispielsweise einen Kommentar erstellt, zum laufenden Prozessschritt zurückkehrt und dann versucht, zum vorherigen Prozessschritt zu navigieren, würde er bei Verwendung einer Previous-Logik versehentlich zum Kommentarschritt anstatt zum vorherigen Schritt in der Sequenz navigieren.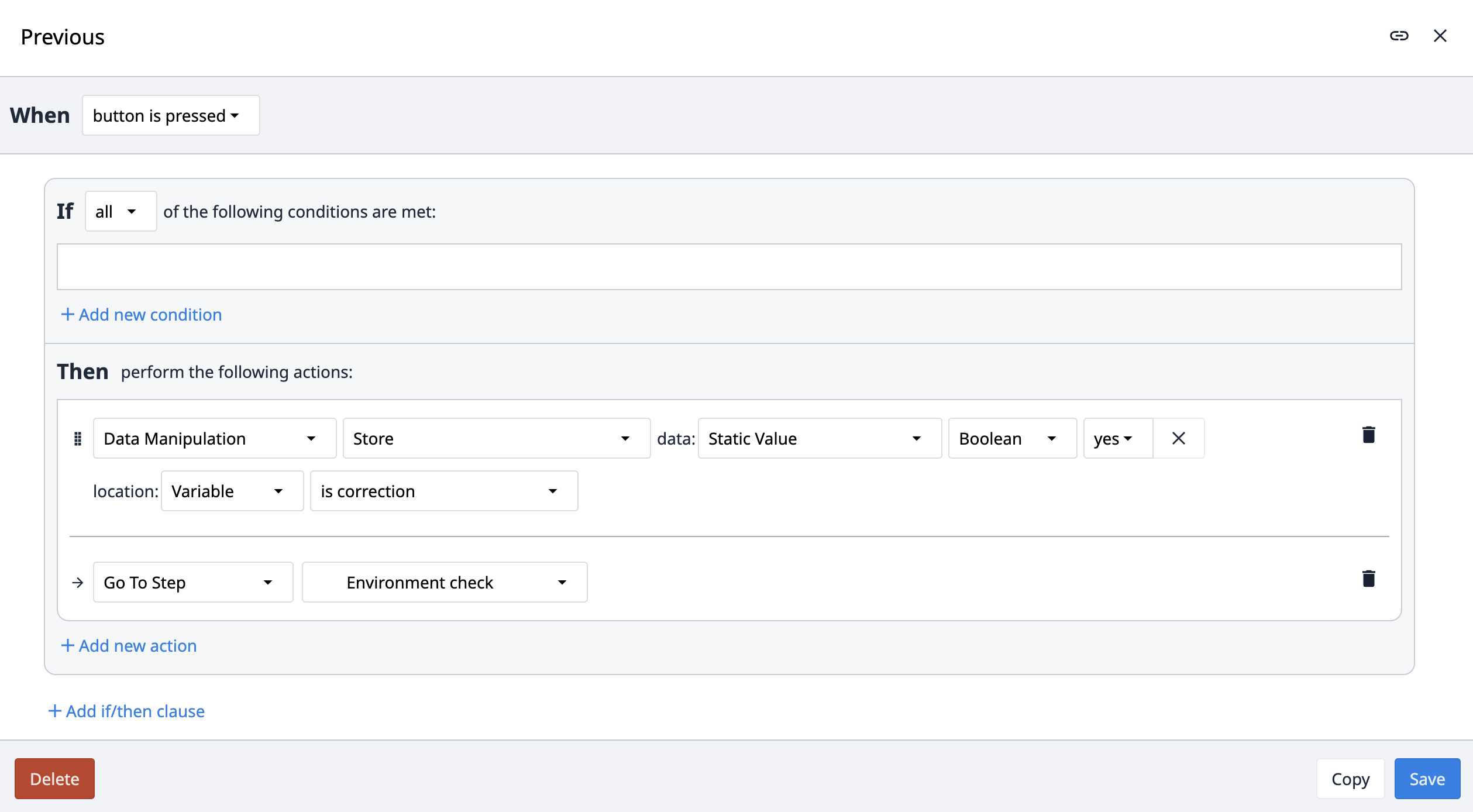
Der Auslöser Gehe zu wird auch in der App-Suite verwendet. Anstatt zum nächsten oder vorherigen Schritt zu gehen, geht dieser Auslöser zu einem bestimmten Schritt, der im Auslöser selbst definiert ist.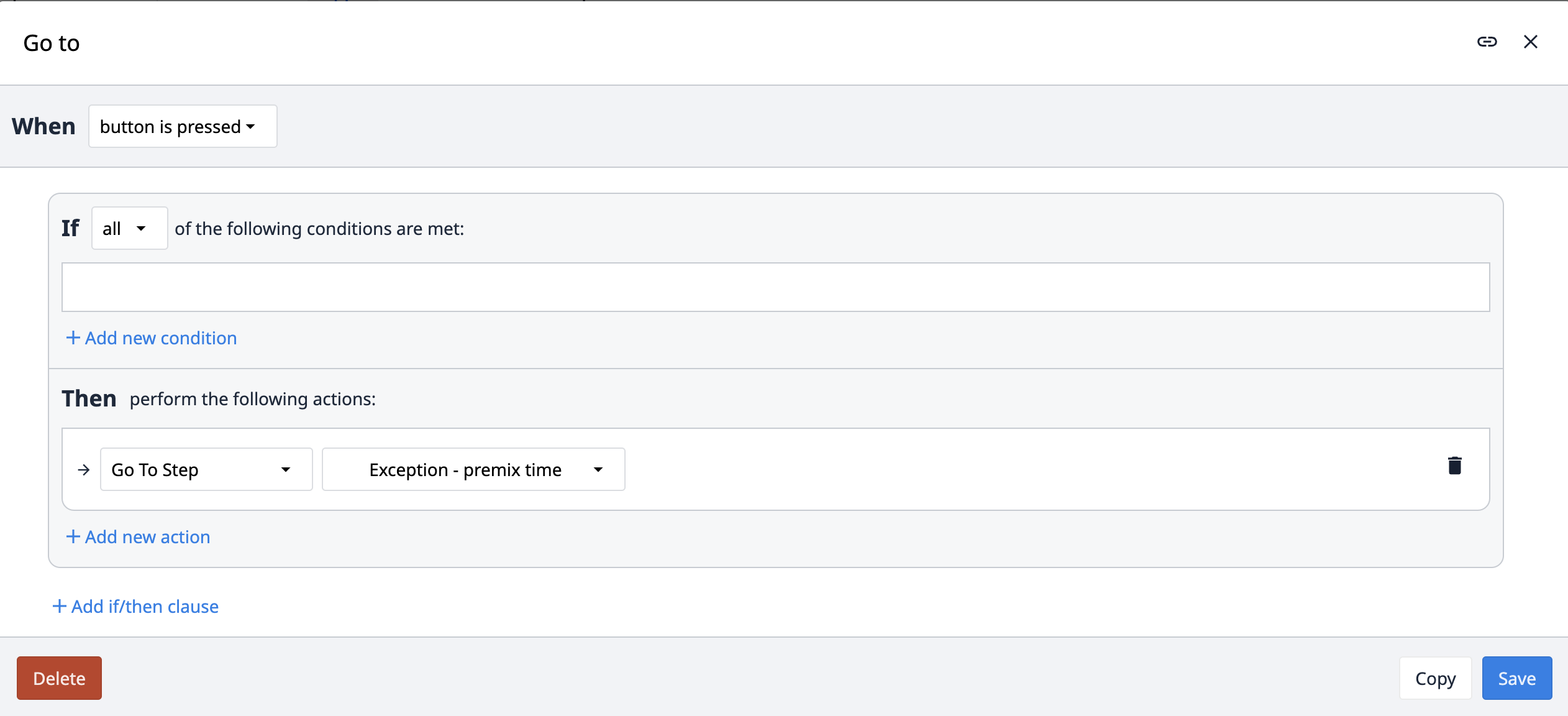
Der Routing-Trigger navigiert die App auf der Grundlage bestimmter Bedingungen zu verschiedenen Schritten. Zu welchem Schritt die App navigiert, hängt davon ab, welche Bedingung erfüllt ist.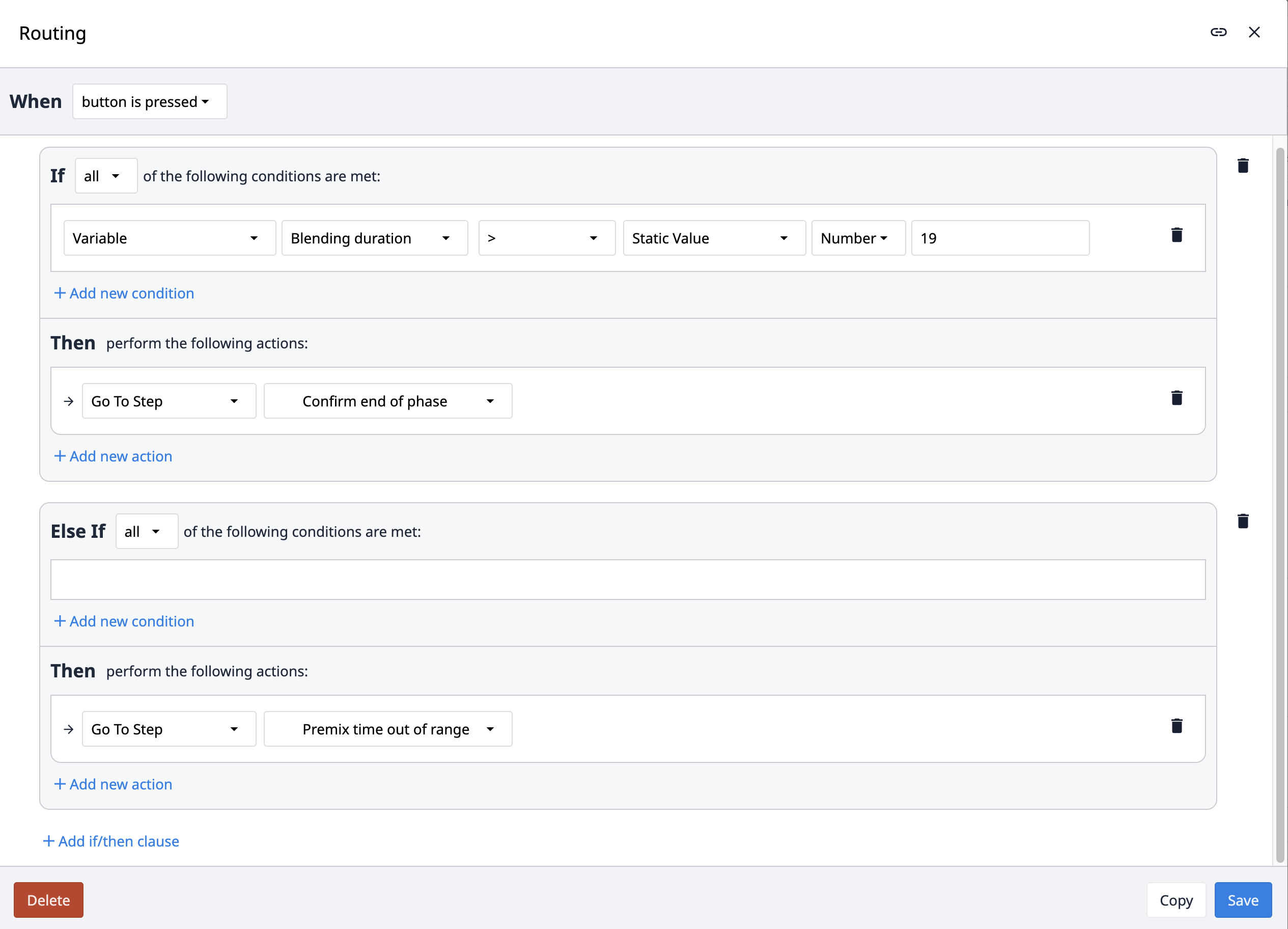
Kommentare, Ausnahmen und Korrekturen
In allen Anwendungen des Composable MES for Pharma haben wir einheitliche Regeln für die Erstellung von Kommentaren, Ausnahmen und Korrekturen verwendet**.** Jede Anwendung enthält eine Schaltfläche " Kommentar melden" als Teil ihres Basislayouts. Wenn der Benutzer auf diese Schaltfläche klickt, wird er zum Schritt "Kommentar" weitergeleitet, in dem er das Problem beschreibt und optional ein Bild beifügt. Wenn Sie auf die Schaltfläche Kommentar protokollieren klicken, erstellt die Anwendung einen Datensatz in der Tabelle Kommentare & Ausnahmen.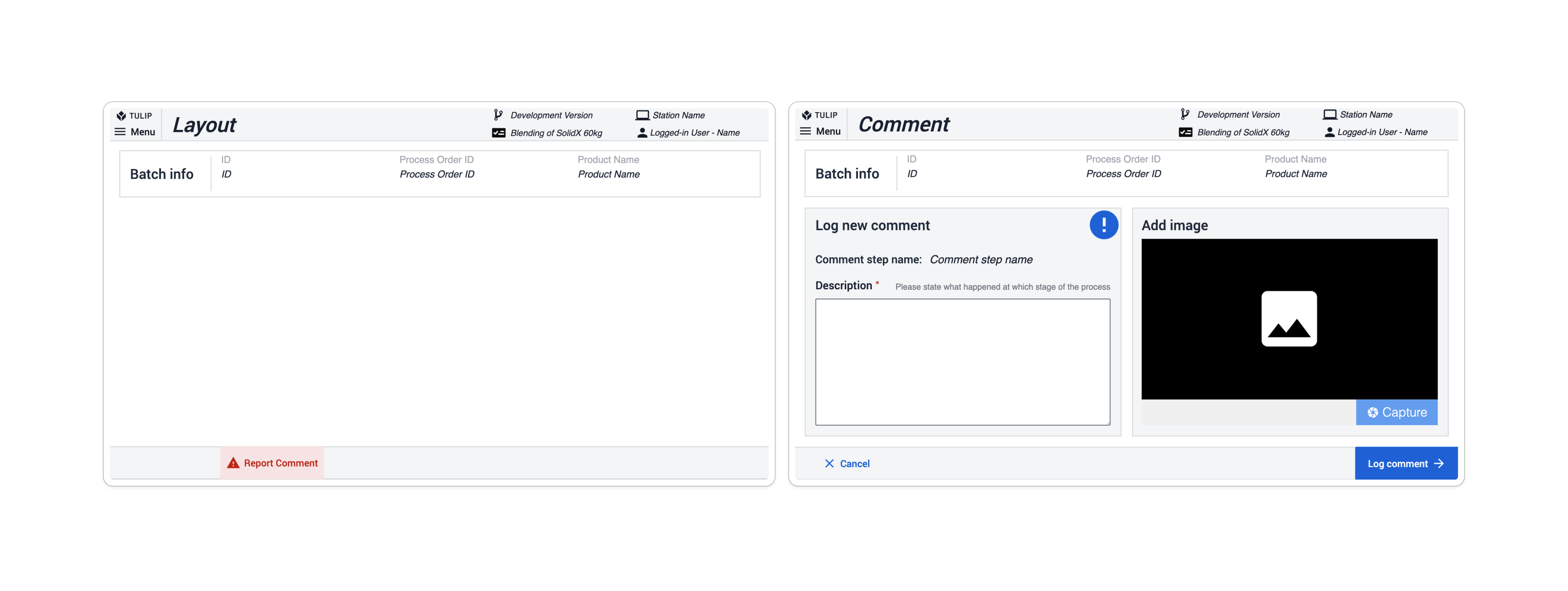
Im Composable MES for Pharma werden Ausnahmen verwendet, wenn eine Abweichung vom Prozess erwartet wird (z. B. wenn ein Inspektionswert im Vergleich zu einem bestimmten Satz von Grenzwerten ausfällt). App-Suites enthalten Ausnahmeschritte auf der Grundlage des Auftretens, meist nach dem Schritt, bei dem eine Abweichung auftreten könnte. Im Schritt der potenziellen Abweichung prüft eine in die Navigationsschaltfläche integrierte Bedingung, ob der vorhergesagte Prozess wie erwartet abläuft. Ist dies der Fall, wird der Ausnahmeschritt übersprungen; ist dies nicht der Fall, navigiert die App zum nächsten Schritt, dem Schritt " Ausnahme". Hier muss der Benutzer eine Ausnahme erstellen, um den Prozess fortzusetzen.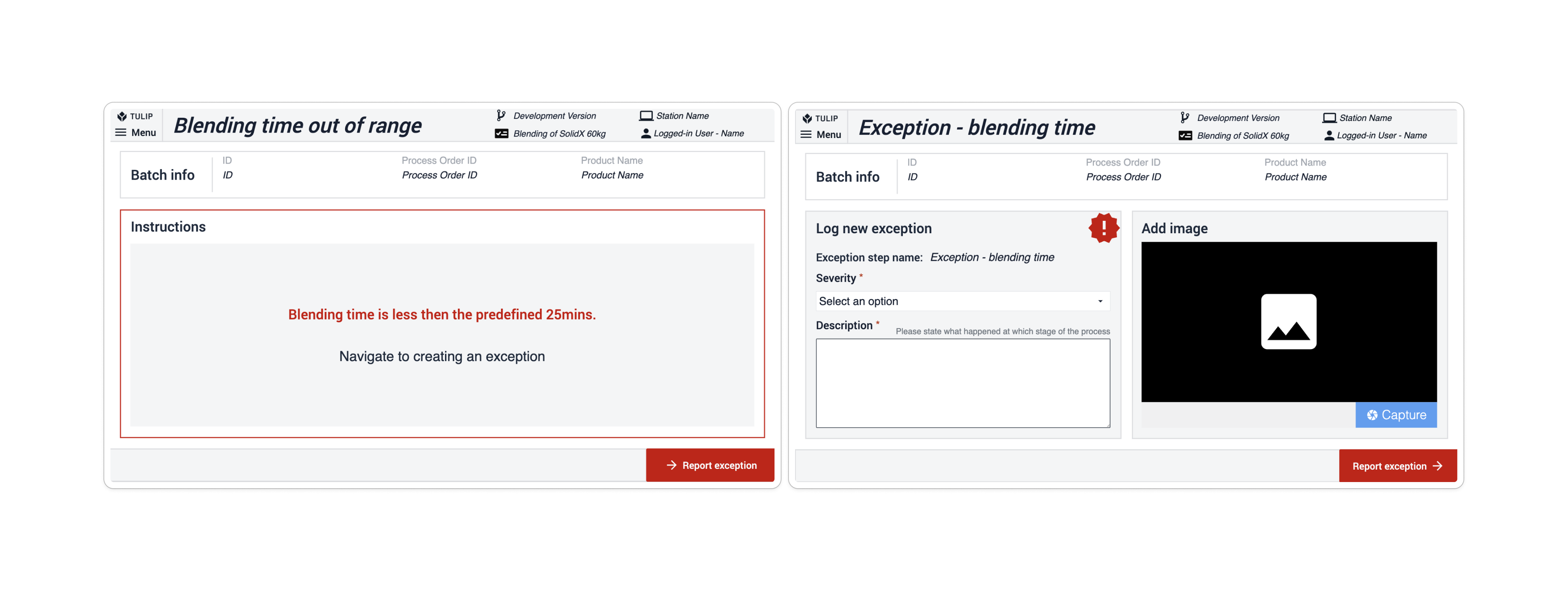
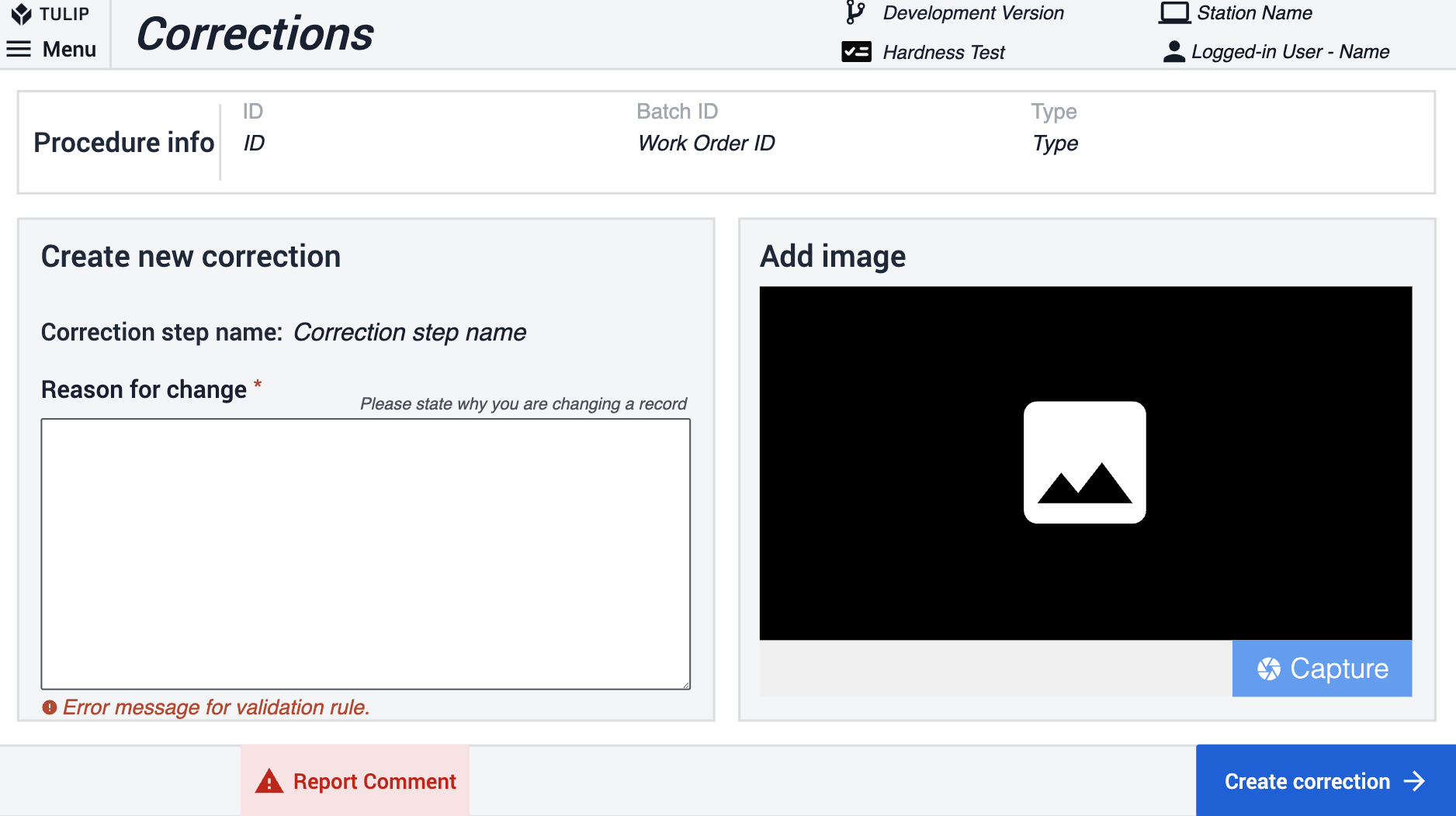
Korrekturen innerhalb der App-Suite werden von den App-Benutzern verwendet, um Gründe für das Zurücknavigieren oder das Ändern zuvor eingegebener Informationen zu erfassen. Eine boolesche Variable Is correction wird in der gesamten Suite für Schritte verwendet, bei denen eine Navigation zu den vorherigen Schritten möglich ist. Diese Variable ist standardmäßig auf Nein gesetzt. Die Variable Is correction wird auf true gesetzt, wenn der Benutzer innerhalb der App zurücknavigiert.
Wenn die nächste Schaltfläche angeklickt wird, wertet die App die Variable Is correction aus. Ist sie falsch, wird mit dem nächsten Schritt fortgefahren. Ist sie wahr, speichert die App den Schrittnamen und leitet zum Korrekturschritt weiter. Im Schritt Korrekturen müssen die Benutzer einen Grund für die Informationsänderung angeben und können optional ein Bild hinzufügen. Wenn Sie auf die Schaltfläche Korrektur erstellen klicken, protokolliert die App dies in der Korrekturtabelle, setzt die Variable Ist Korrektur auf Nein zurück und navigiert zurück zum Schritt, der auf dem zuvor gespeicherten Schrittnamen basiert. Die Korrekturen können durch Einsichtnahme in die Tabellendatensätze überprüft werden.
Datenvalidierungen in Anwendungen
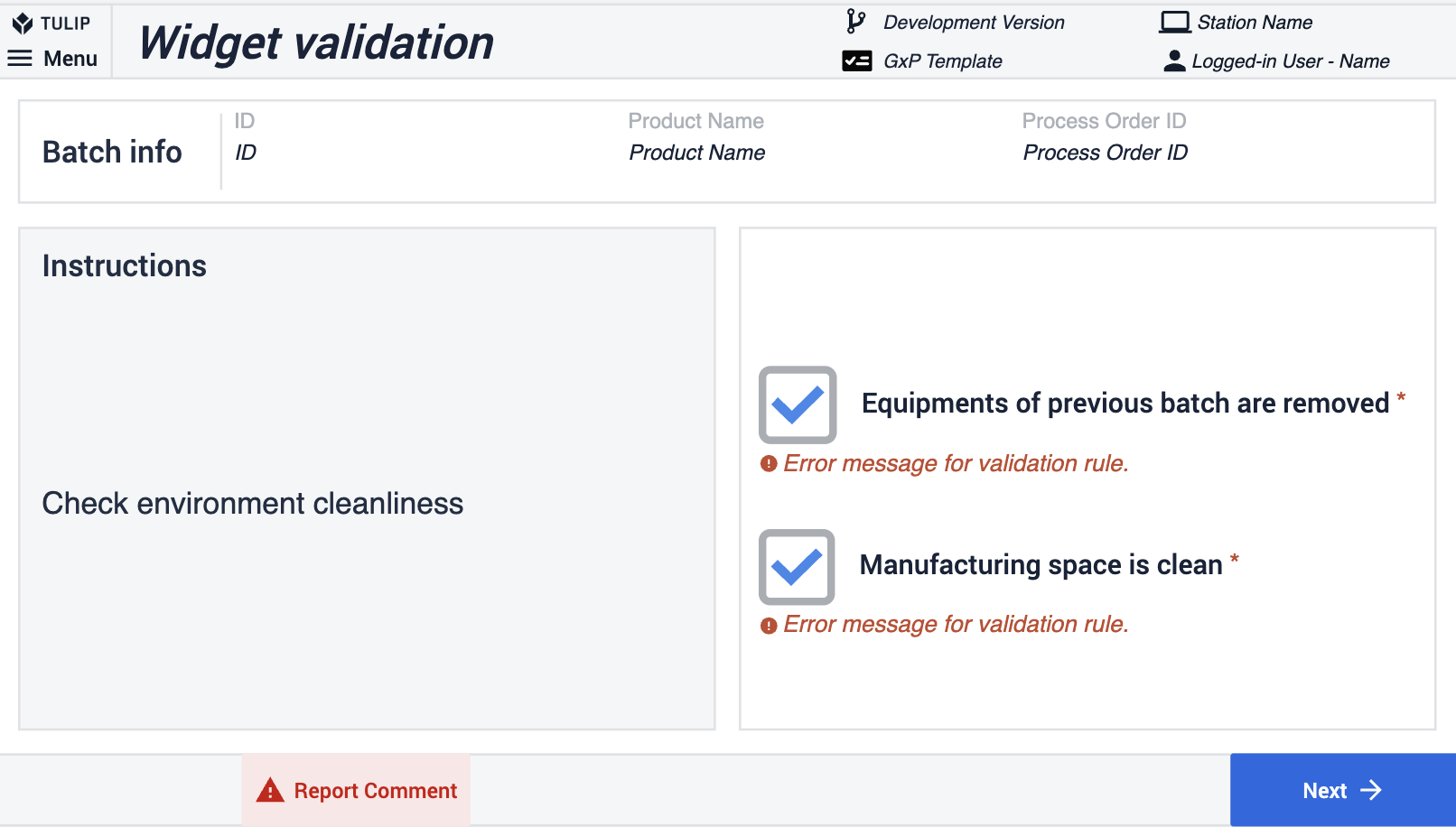
Im Composable MES for Pharma gibt es drei verschiedene Methoden zur Validierung von Informationen in Anwendungen. Die erste Methode besteht in der Verwendung der Validierungsregeln des Widgets. Im folgenden Beispiel bleibt die Schaltfläche Weiter deaktiviert, wenn das Kontrollkästchen nicht aktiviert wurde.
Die zweite Methode demonstriert die Verwendung von Validierungsregeln in Zahleneingabe-Widgets. Zunächst wird eine Regel angewendet, die einen Bereich festlegt: Wenn die eingegebene Zahl außerhalb dieses Bereichs liegt, bleibt die Schaltfläche deaktiviert. Zusätzlich schreibt eine weitere Regel vor, dass die Zahleneingabefelder ausgefüllt werden müssen; die Schaltfläche ist inaktiv, bis dies geschieht.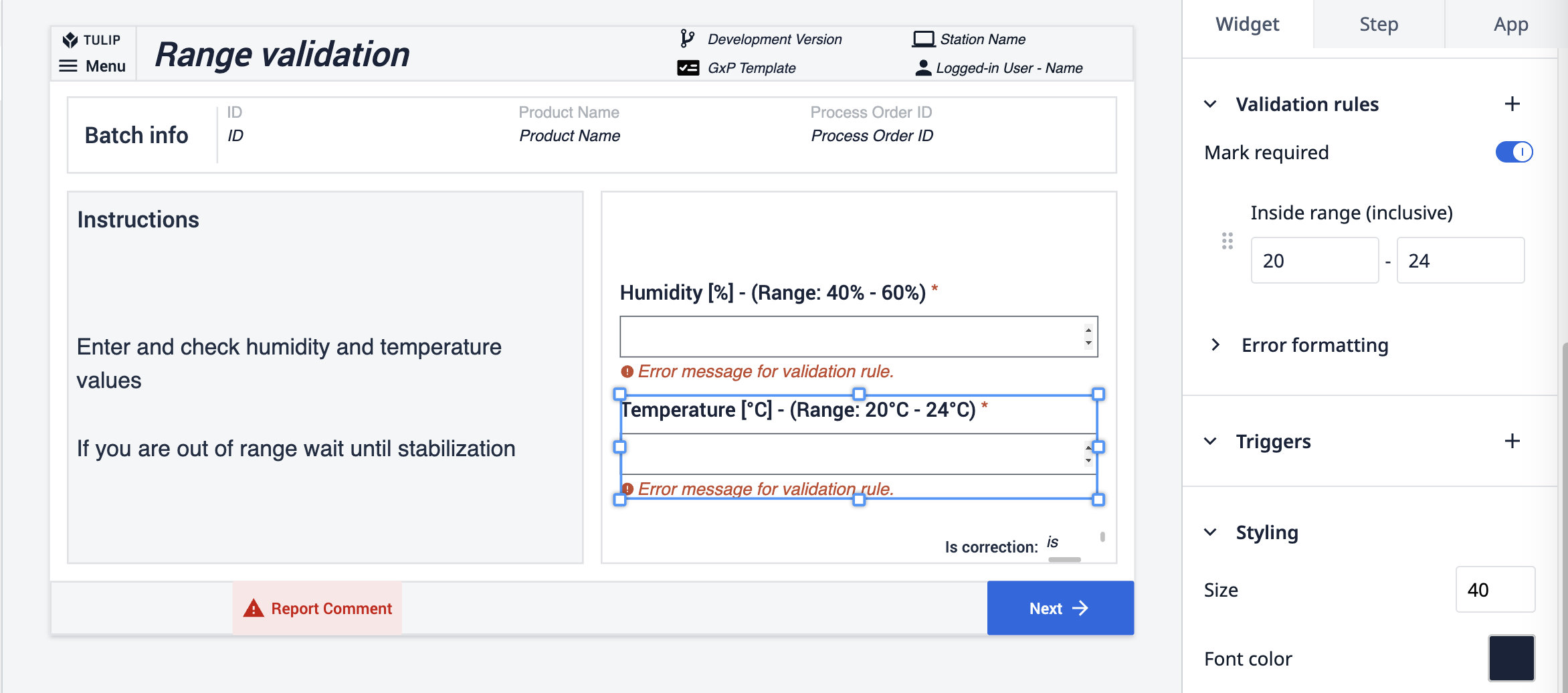
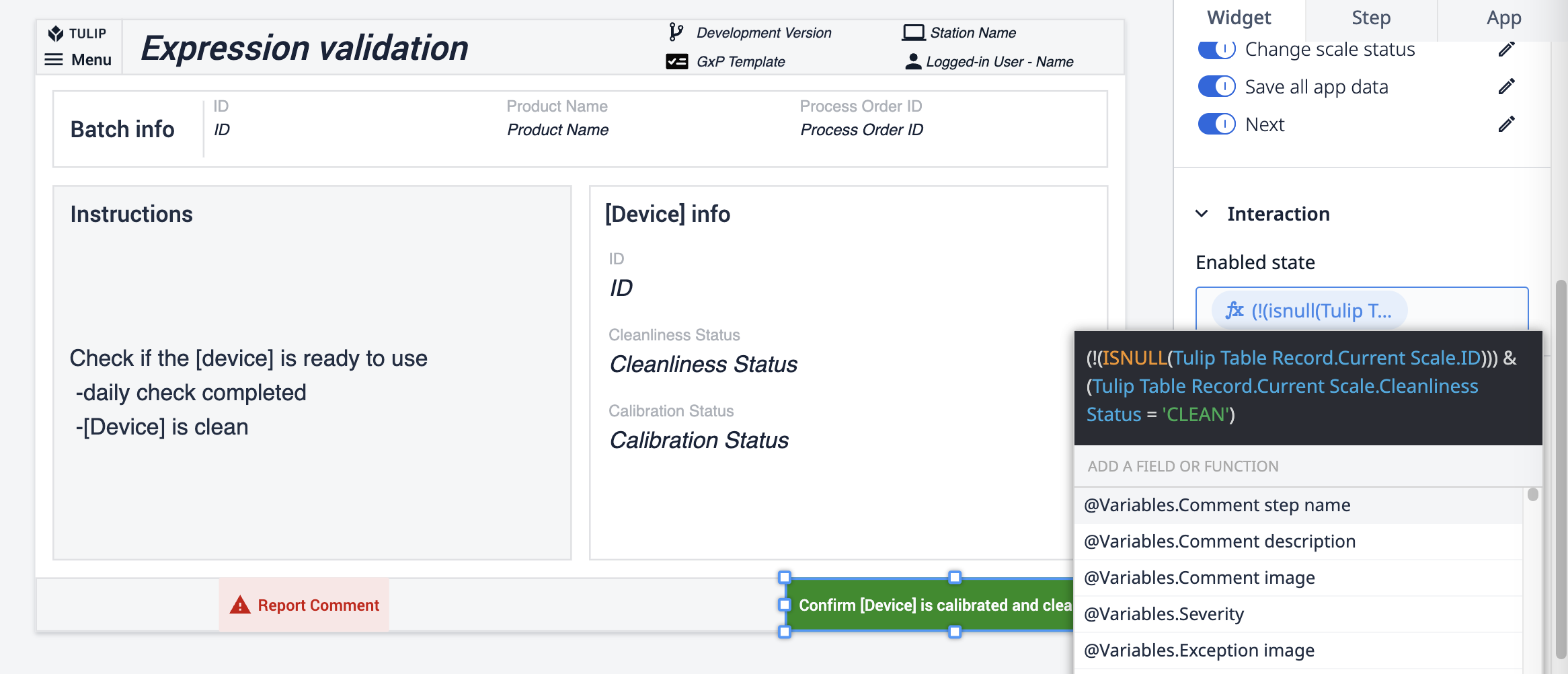
Die letzte Methode wird direkt auf die Schaltflächenaktion angewendet. Ein mit der Schaltfläche verbundener Ausdruck prüft den Sauberkeitsstatus der Waage. Wenn der Status nicht CLEAN ist, bleibt die Schaltfläche deaktiviert.
Elektronische Unterschrift Widget
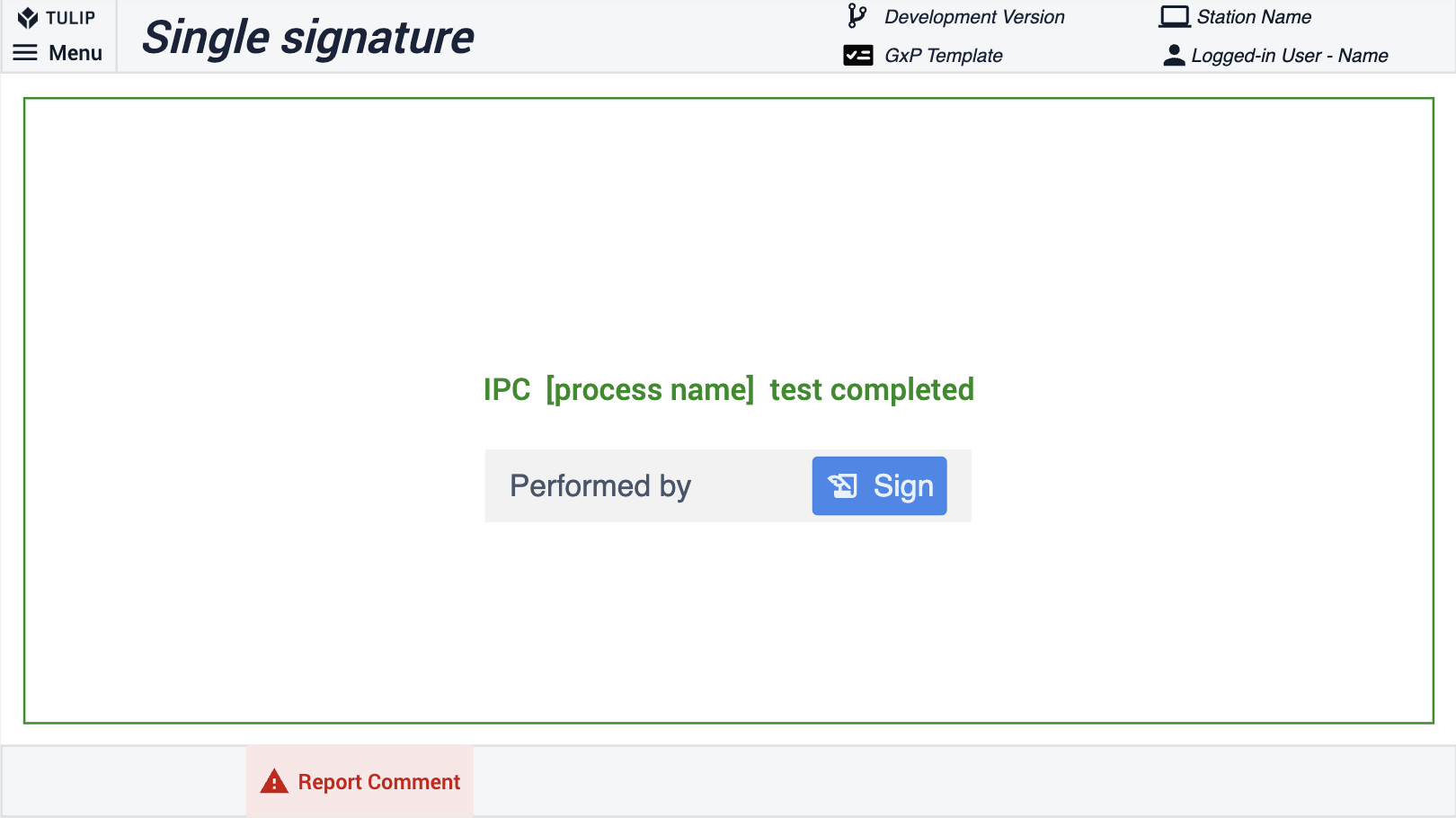
Um die Einhaltung der Vorschriften einfach und nativ in die Tulip-Plattform zu integrieren, bietet das Widget für die elektronische Unterschrift eine Möglichkeit, Daten innerhalb von Tulip zu unterschreiben. In Übereinstimmung mit 21 CFR Part 11 bietet dieses Widget eine rechtsverbindliche Darstellung einer physischen Unterschrift. Die Unterschrift ist unveränderlich und wird in den Daten Ihrer Anwendung Completion gespeichert. Sie kann nicht neu zugewiesen, übertragen oder gefälscht werden. Im Composable MES for Pharma verwenden wir das Widget Elektronische Unterschrift, das bei angemeldeten Benutzern die Unterschrift durch einen Abschlussdatensatz ersetzen kann. Das Signatur-Widget wird typischerweise für Check-Bys verwendet. Im folgenden Beispiel kann der Benutzer mit einem einzigen Unterschriften-Widget den Prozess abzeichnen. Zur zusätzlichen Qualitätssicherung können Sie zwei Unterschriften in einem einzigen Schritt platzieren, um zu zeigen, wer eine Aktion durchgeführt und überprüft/genehmigt hat.
Widget "Datensatzverlauf
Das Record History Widget ermöglicht eine streng GxP-konforme Überprüfung der Daten. Das Widget ist so konfiguriert, dass alle Änderungen angezeigt werden, die an einem bestimmten Artefakt (Tabellendatensatz) wie einer Charge oder einem Material vorgenommen wurden. Um die Verwendung des Widgets zu ermöglichen, verwenden Sie in Ihren Prozessanwendungen denselben Satz von Tabellen und fügen Sie in der Überprüfungsanwendung die Tabellen zum Widget im verknüpften Platzhalter hinzu.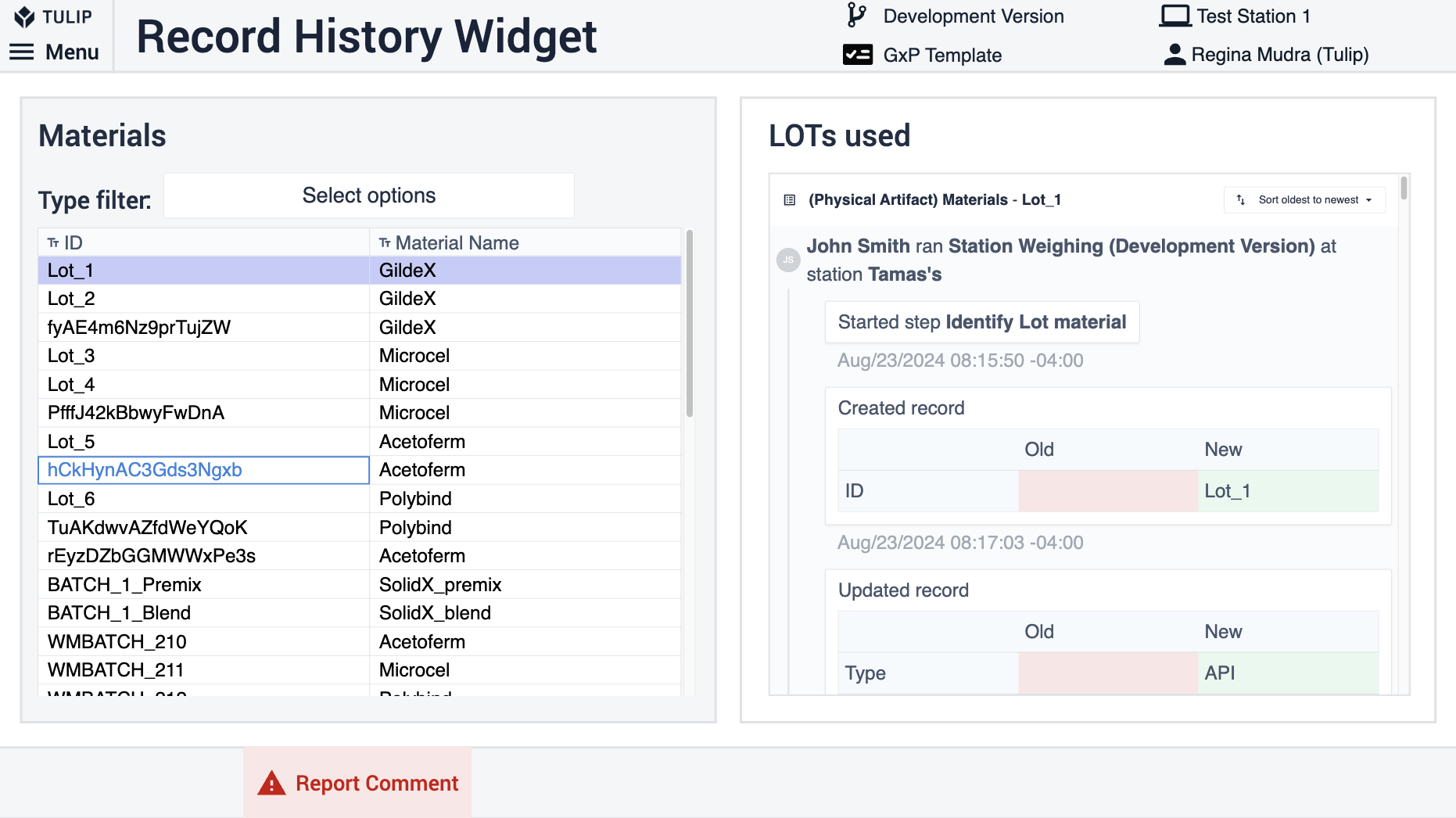
Konfiguration
In-App-Hilfe
Alle Anwendungen im Composable MES for Pharma enthalten eine In-App-Hilfe. Dies sind kurze Beschreibungen der erforderlichen Einrichtungsschritte und Tipps für den App-Builder zur Unterstützung weiterer Anpassungen. Lesen Sie nach dem Herunterladen der Anwendung diese Anweisungen und löschen Sie sie anschließend, bevor Sie die Anwendung ausführen.
Zusätzliche Ressourcen
App-Beispiele und Templates
Composable MES for Pharma enthält App-Beispiele und -Vorlagen. App-Beispiele sind Anwendungen mit integrierten, vordefinierten Informationen, die dem Benutzer helfen, die Anwendung zu verstehen und sie zu testen oder zu demonstrieren. Jede Anwendung repräsentiert einen bestimmten Prozessschritt, der in Ihrer Produktion vorkommt. Vorlagen dienen als Ausgangspunkt, indem sie wiederverwendbare Bausteine bereitstellen, die Sie leicht für eine breite Palette von Prozessen anpassen können. Durch die Kombination von Anwendungsbeispielen und Vorlagen im Composable MES for Pharma können Sie Ihre eigenen Versionen dieser Anwendungen leicht verstehen, testen und bereitstellen, um Ihren Entwicklungsprozess zu beschleunigen. Nach der Anpassung dieser Vorlagen an Ihren Betrieb können Sie sie standardisieren oder verteilen und lokale Konfigurationen zulassen, um globale Bereitstellungsprojekte zu beschleunigen.
Referenzen
Composable MES für PharmaProductionManagement app suiteCommonData Model für PharmaElectronicLogbook
