Imagine you are a quality manager who dedicates significant time to reviewing batch records. During the review process, you find that records contain errors and inconsistencies. Some errors are due to operator handwriting. Others contain empty fields which can be difficult to correct (such as a timestamps or missing signatures). These errors introduce risk, both from auditors and to end-users of your products. In an effort to make data collection easier and standardize data formats, you build a digital history record solution. In some places, there are hard controls to prevent operators from continuing the batch in critical to quality situations. Elsewhere, the operator is required to document an exception before continuing. Exception management workflows augment digital history records and can integrate with other software to seamlessly track process deviations, document investigations, and provide traceability to other systems of record.
A digital history record is a digital replacement for the traditional paper-based system used by manufacturers of pharmaceuticals or medical devices. It is used in regulated industries to document and archive the manufacturing history of their products and to comply with regulatory requirements.

There are two types of digital history records that we’ll discuss:
- Electronic Device History Records (eDHR)
Used in the medical device and diagnostics industry - Electronic Batch Records (eBR)
Used in pharma, biotech, cell and gene, food and beverage, and cosmetics
With digital history records, you can streamline compliance procedures, improve operational efficiency, and ensure safety and readiness in the production of pharmaceuticals and medical devices.
Digital history records offer several benefits for various personas involved in the data capture and review process. The following table summarizes how this is accomplished:
| Persona | How they benefit |
|---|---|
| Manufacturing operators | Capture data in real-time to create an accurate auditable trail, in a manner that is more efficient and user-friendly than paper. |
| Quality assurance/Compiance personnel | Review and approve records to ensure quality and safety for the market. They can also demonstrate control to auditors with available, easy-to-find, and accurate information. |
| Quality control | Review records to track quality issues and identify root causes. This information drives continuous improvement of operations. |
Impact and Requirements
Traditional approaches to history records involve much more risk than digital history records. Manual entry leaves room for error and the physical record limits access and prolongs the approval process. Here are the ways digital history records can solve these problems and others:
- Operator transcription/calculation errors
- Digital records eliminate unclear handwriting
- Automatically calculate values
- Inaccurate data entry
- Standardized data entry formats
- Data input validation to ensure values are within spec
- Errors are not caught until signature or quality review
- Automatically flag incorrect information upon input
- Error messages that inform operators of incorrect inputs
- Prolonged process due to one physical record that is passed around
- Parallel processes that capture relevant data
- Automatic data compilation to provide full history
- Line-by-line quality approval that takes a long time
- Choose what data to review based on what is most important
- Review-by-exception capability
Solving particular issues is dependent on your operation. Digital history records can also feed into company objectives, as detailed in the table below:
| Company objective | How to accomplish | Relevant KPIs |
|---|---|---|
| Enhance traceability and transparency | Timestamps, audit trails, FDA 21 CFR Part 11 and EU Annex 11 compliant electronic signatures | Audit readiness (critical data is available and easily accessible), Streamline CAPA with documentation and quality issue tracking |
| Improve quality control | Detect, triage, and handle anomalies and deviations from SOPs, Eliminate transcription errors and increase compliance by reducing human error | Increase first pass yield, decrease transcription errors, forced compliance (i.e. the system will automatically generate a deviation) |
| Optimize productivity | Accelerate batch release with review-by-exception workflows | Increase throughput, OEE (accurate machine uptime can enhance OEE calculations), faster batch review allows a decreased overall lead time and faster release to market |
This use case is recommended for eDHR and eBR operations. A digital history record is a complex solution because it must fulfill every requirement for compliance purposes and quality standards. This use case has a medium/high complexity, depending on your specific operation. To build an optimal digital history record solution, you must have a solid foundation of app building in Tulip and the Tulip common data model.
Validation is an important part of digital history records. Take the Tulip University course on Quality and Validation.
How to Get Started
In order to create an optimal digital history records solution, you need to understand your process, including the data flow from external sources. Then, you need to establish a clear data model for what information is stored where.
Map out the process
When you map out the process, consider the following questions:
- What data is captured during the process?
- What in-line checks should be included in the DHR?
- What data do you need to show in the history records?
If you use an external system, make sure there is a clear path of data flowing into Tulip. This includes process data from MQTT or order/material data from an ERP.
Adapt to a digital structure
Your existing paper batch records (or similar) should serve as the blueprint for a digital solution. This does not mean that the digital records should look exactly like the paper ones. Rather, the information of each batch or device is important to capture in Completion records.
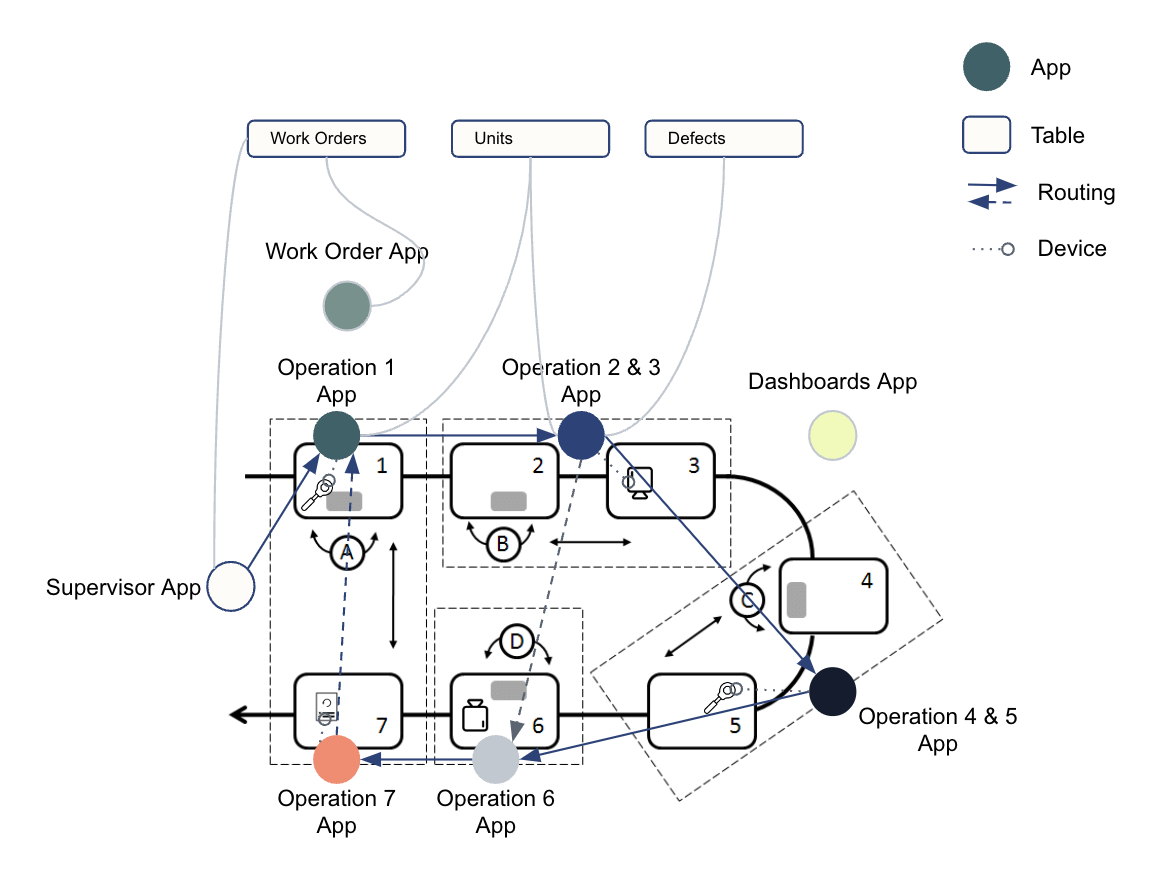
You should map out what functions need to happen, which apps will accomplish tasks, and the storage and transfer of data in between apps. This helps visualize your physical process and how it transitions to a digital solution. See the example below:
Establish a data model
A data model organizes information and designates it into different categories. In the Tulip Common Data Model, there are two primary categories of Tables:
- Physical artifacts - tangible objects or components that are used or produced during operations.
- Operational artifacts - non-physical elements or components that enable or support operations. These are often found as printed travelers or tickets.
Here are a few best practices when it comes to building a data model:
- Ensure there is only one “primary artifact” (i.e. batch, work order, device, etc.) that serves as the placeholder which all data is captured against in completion records
- Use table ID naming conventions that are human-readable (no random strings)
- Avoid building activity history tables, as this information should be stored in completions
Learn more about how to use a common data model.
Any process data relevant to a specific batch (timestamps, conditions, operator info, Electronic Signature) should be stored in app completion data. This ensures the data is immutable and avoids duplication.
Read about best practices for GxP data collection.
Collect and view history records
To collect data, use a mix of tables in your common data model and app completion records. The collection happens in your process apps, like work instructions, weigh and dispense, or any other activity that generates data.
When you need to view history records, you’ll need to use a mix of the interactive table widget, Analytics, and the record history widget.
The record history widget is essential for reviewing completion records:
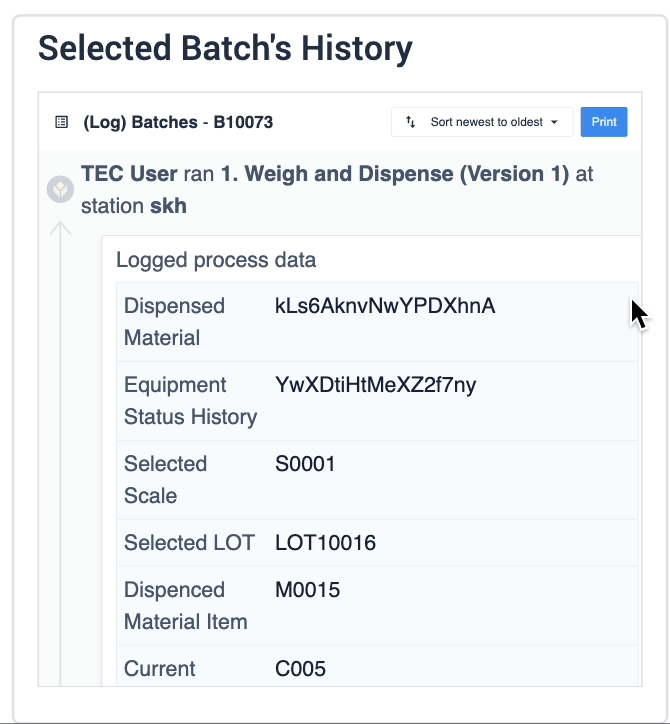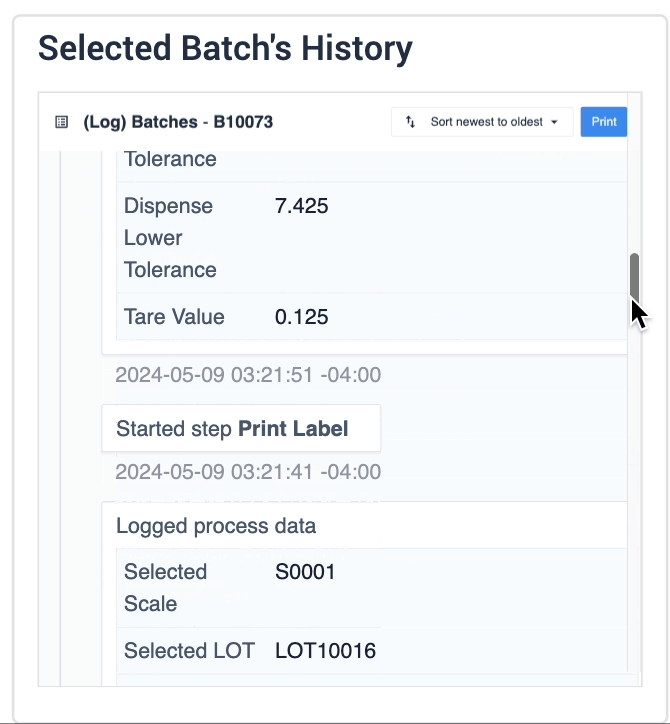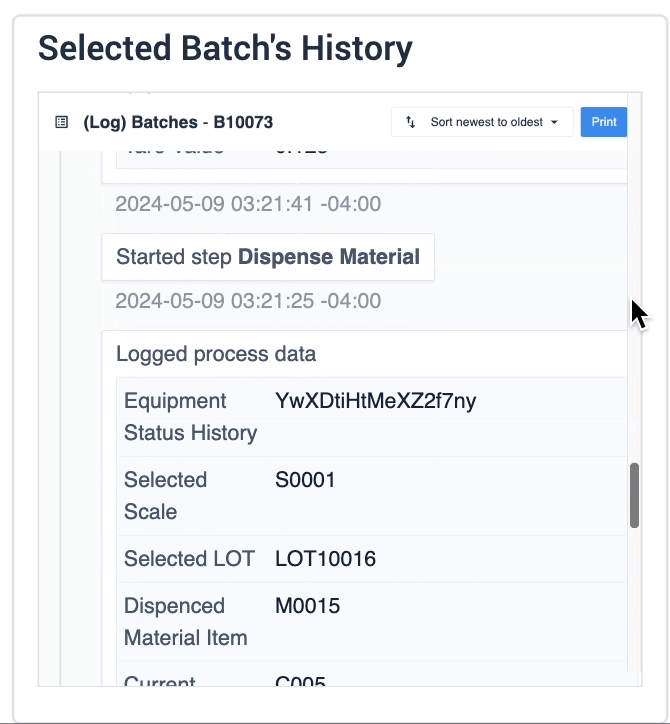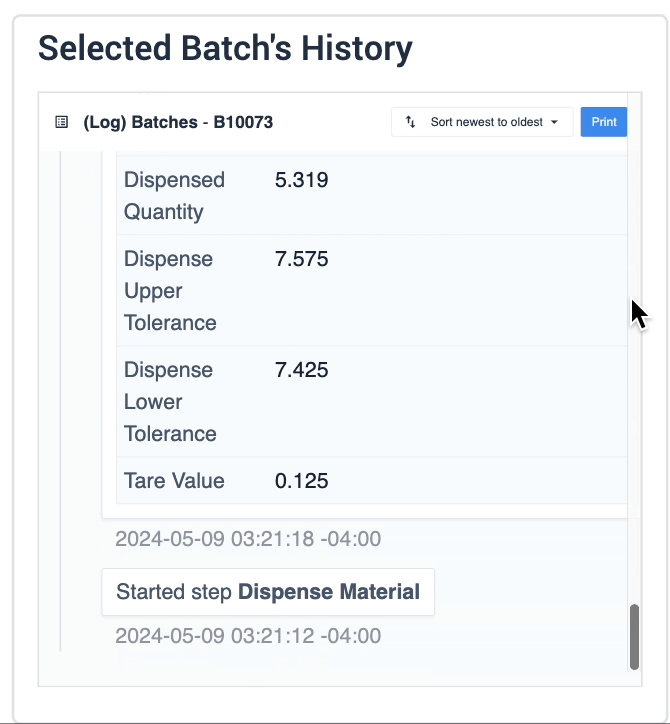
In the example below, data of the selected device is displayed in the table record widget on the left. The process data is shown in the record history widget on the right of the step.

The second example uses a similar step layout, but the batch selection in the table record widget is an interactive list the user can choose from. The record history widget displays batch information based on the user’s selection.

You can intersect a digital history records solution with any other use case that collects data, such as logbooks, weigh and dispense, production tracking, etc. Use the information collected in other apps to build a holistic view of a batch.
Tulip Resources
Whether you want to learn more about Tulip features to build a digital history records app or you want to use Tulip’s ready-made templates, we have the tools to help you get started.

.gif)