Quality events in the Frontline QMS
Quality events are critical occurrences in manufacturing that highlight deviations from expected standards. These events help manufacturers identify, address, and prevent quality issues, ensuring compliance with regulations and maintaining customer trust. While various types of quality events exist, they can generally be categorized into those more closely handled on the shop floor and those managed by back-office quality teams.
Common Types of Quality Events
Defects
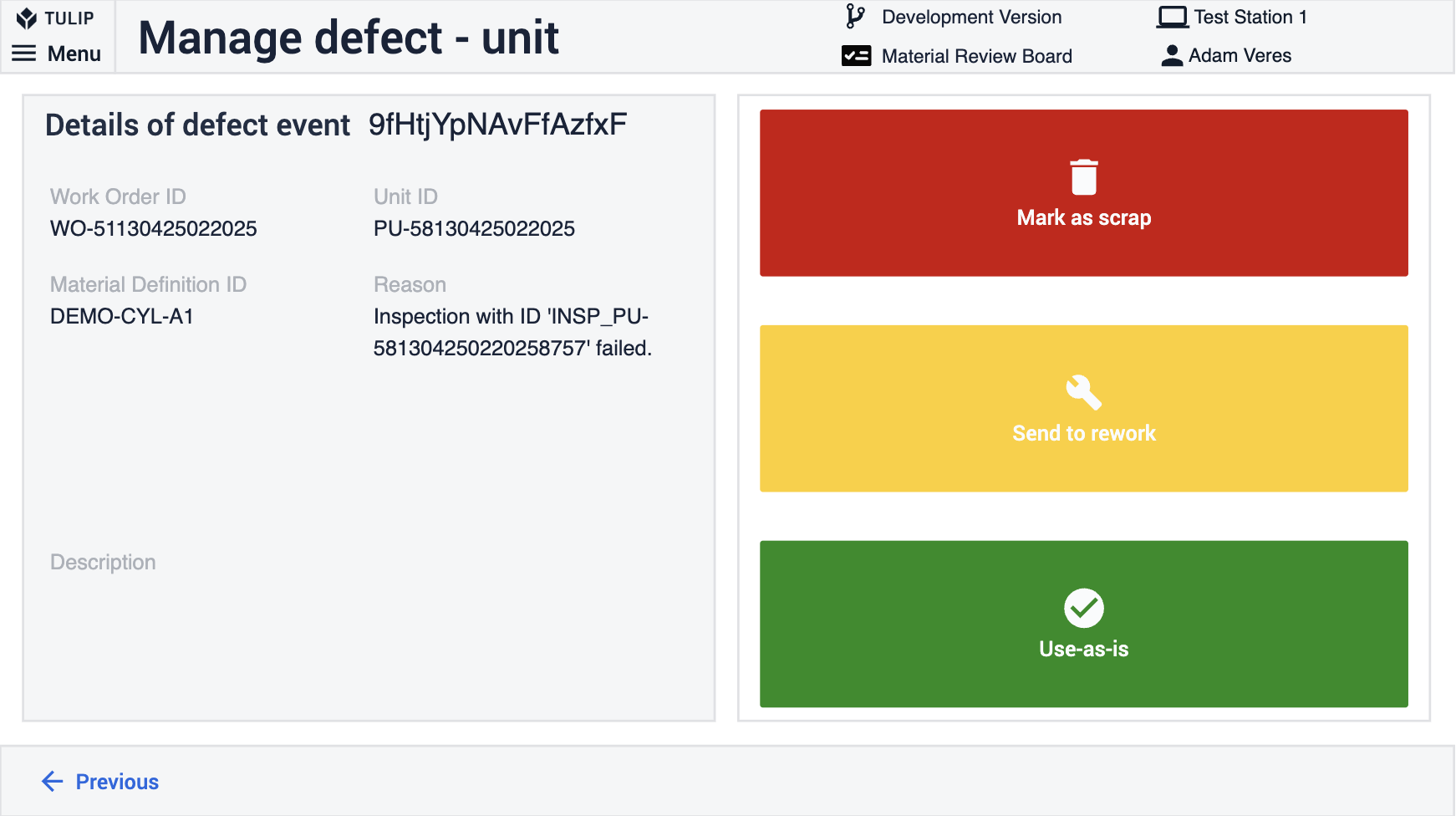
Defects occur when a product unit does not meet quality specifications. Defective units may be scrapped, reworked, or flagged for further inspection. Proper defect tracking helps manufacturers reduce waste, improve processes, and maintain product quality.
Corrective and Preventive Action (CAPA) Events
CAPA events stem from defects or audit findings, requiring investigation and corrective actions to prevent recurrence. These events are vital in improving processes and ensuring regulatory compliance.
Audit Findings
Audit findings arise from quality audits and indicate non-compliance issues. Unlike defects, they often pertain to procedural adherence rather than physical product quality. These findings could come from:
- Internal audits (company-wide assessments)
- Regulatory audits (compliance with industry or legal standards)
- Supplier audits (ensuring vendor compliance)
Notice of Escape (NOE)
A Notice of Escape occurs when a defective product has already been sent to a customer. It necessitates urgent action to mitigate risk, possibly including customer notifications, recalls, or rework processes.
Re-Inspection Requests
If a supplier issues a NOE, manufacturers may initiate a re-inspection request to verify incoming product quality. This ensures that any defective parts do not compromise production.
Out-of-Calibration Events
These events occur when a measurement tool is suspected of being out of calibration, meaning any units measured with it may require re-inspection. Managing calibration effectively prevents the risk of passing incorrect products.
Frontline quality events managed by Tulip Frontline QMS
Defect detection and inspection processes are crucial for frontline quality management. A robust defect-tracking system ensures:
- Early identification and isolation of defective units
- Efficient workflows for rework, repair, or scrap decisions
- Real-time data for quality improvement
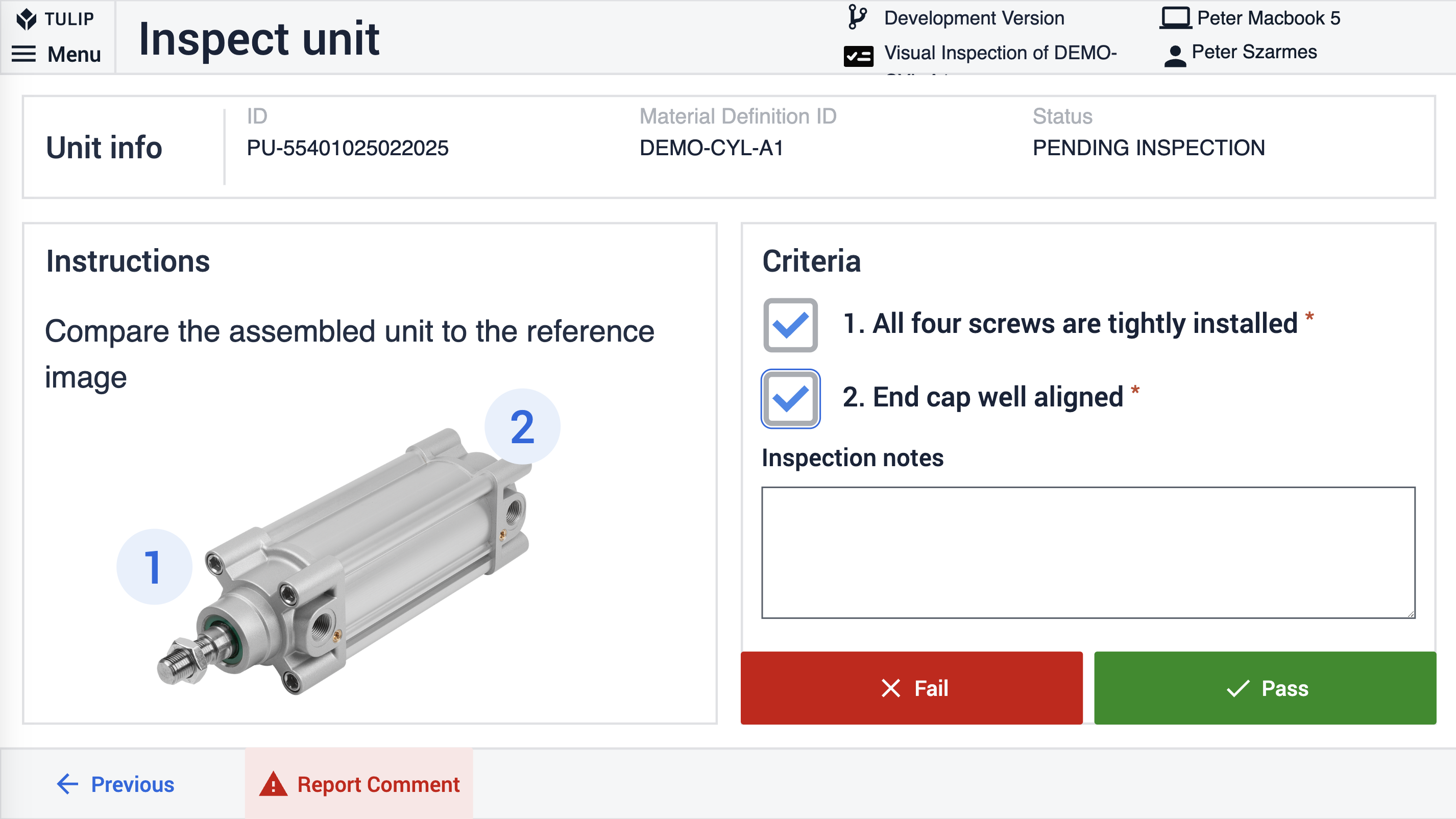
The Tulip frontline QMS provides the MRB app and the Inspection Plan Creator together with the Inspection Execution to help frontline workers effectively handle defects and quality inspections.
Inspection requests, including re-inspections due to supplier NOEs or internal checks, help manufacturers maintain high product standards. By digitizing inspection processes, manufacturers reduce manual errors and improve traceability.
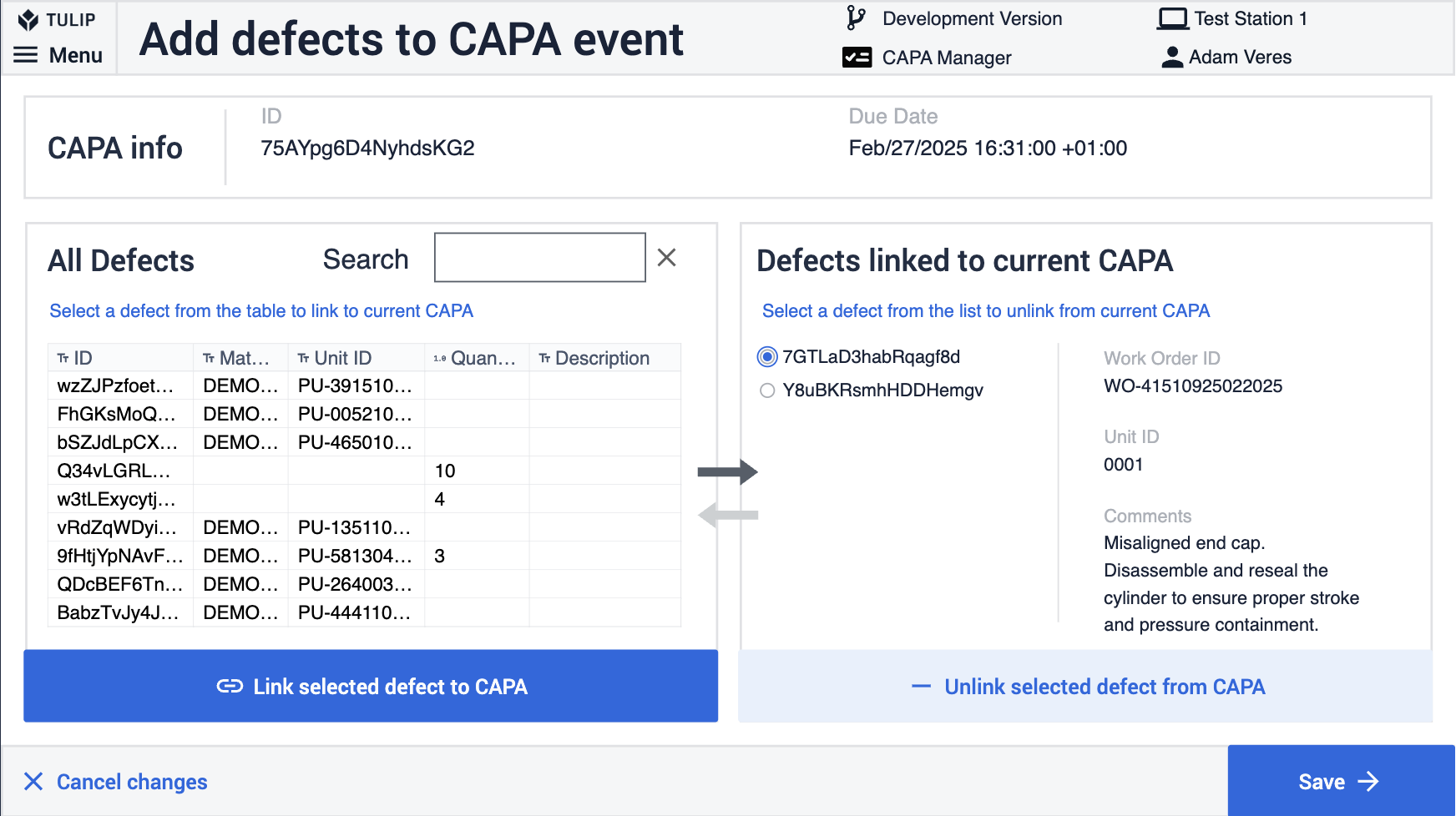
The CAPA process is also very important on the shop floor. Many CAPA events can be associated with defects. This process ensures that product or process flaws are corrected and prevented in a rigorous manner.
A well-structured CAPA process helps manufacturers:
- Identify root causes of defects and audit findings
- Implement corrective actions to prevent recurrence
- Maintain compliance with industry standards
By capturing CAPA events digitally on the shop floor, manufacturers can ensure seamless collaboration between operators, engineers, and quality teams.
Traditional quality management systems focus more on back-office compliance and long-term process improvements. However, a frontline quality management system—such as this suite of applications—offers key advantages beyond this (especially when integrated):
- Real-time quality tracking: Immediate capture and resolution of quality events
- Digital workflows: Standardized, paperless defect and inspection management
- Closeness to production: Seamless connection between quality processes and shop floor operations
- Operator empowerment: Providing frontline workers with guided workflows to ensure compliance and efficiency
- Faster resolution times: Reducing delays in defect handling and CAPA implementation
While comprehensive quality management requires both frontline and back-office solutions, Tulip’s Frontline Quality Management Suite streamlines defect tracking, CAPA management, and inspection workflows. This empowers manufacturers to react swiftly to quality events, reducing risks and enhancing overall operational efficiency. With Tulip’s no-code platform, manufacturers can also extend their quality solutions beyond standard processes, tailoring them to their unique needs and driving continuous improvement.